Raman Spectroscopy and Virus Research
George J. Thomas
Professor Emeritus
School of Biological and Chemical Sciences
University of Missouri – Kansas City
Kansas City, MO 64110
thomasgj@umkc.edu
I was first introduced to the King laboratory in 1978, when I received from Jonathan a letter inviting me to visit M.I.T. and discuss with his research group the prospects of applying laser Raman spectroscopic methods to the dsDNA bacteriophage P22 and its constituent proteins. At that time, we had applied Raman methods only to ssDNA filamentous phages and ssRNA plant and bacterial viruses. Aware of the stellar reputation of the King group and the quality of their published work, I was eager to visit the lab and explore collaborative opportunities. What was striking to me was Jon’s familiarity with the language and methodologies of physical chemistry in general and molecular spectroscopy in particular. He had a solid grasp of the fundamentals of Raman scattering spectroscopy of biomolecular systems, a quality I had not encountered in my previous interactions with molecular biologists. We decided to begin a collaboration that led to a long-term research partnership and a close personal friendship. The initial fruit of this collaboration was an exploratory 1980 publication (Fish et al., 1980), followed by more in-depth studies of the mature P22 virion, its precursor particles and constituent proteins (Thomas et al., 1982; Sargent et al., 1988).
In 1982 Jon invited me to join his laboratory group for the duration of a sabbatical semester from my professorship in chemistry at U. Mass. Dartmouth. It was an enriching experience. In the laboratory I learned fundamentals of microbiology appropriate to the plating of bacteria, isolation and characterization of phage and phage proteins, and importantly, the many “cultural” differences between the operations of a laboratory of phage genetics and those of chemistry with which I had been familiar. An interesting outcome of these efforts was the isolation of a temperature-sensitive-folding mutant of the P22 scaffolding protein. Jon always provided patient guidance and an atmosphere of warm welcoming into his research group. (On occasion, this warmth extended to the mysterious disappearance of my lunch bag from the office refrigerator!) Our collaboration continued for many years even as I relocated my research laboratory to the University of Missouri School of Biological Sciences (SBS) in Kansas City, where I had been appointed Head of Cell Biology and Biophysics in 1987. I should add that Jon not only supported this academic transition but unfailingly provided letters of support for my grant renewals and for personal promotions and awards in subsequent years.
As our collaborative work progressed, it became apparent that Raman spectroscopy had the potential to provide new insights into the folding pathway of the trimeric tailspike protein of P22. The folding pathway of the P22 tailspike had been a target of the King lab for many years. We set out to obtain a detailed understanding of the unique and remarkably complex Raman signature of the eight cysteine sulfhydryls (C169, C267, C287, C290, C458, C496, C613, C635) of the native tailspike utilizing site-specific mutagenesis. To this end, we characterized a set of tailspike proteins with each cysteine replaced by a serine. The mutant proteins, once folded, were structurally and functionally indistinguishable from wild-type tailspikes, except for their Raman S–H signatures, which documented the existence of five distinguishable hydrogen-bonded states of buried cysteine sulfhydryls in the native tailspike (Figure 1). Of particular importance is the extremely robust hydrogen bonding of Cys613, stronger than that observed in any other protein to date and implicated in the tailspike folding pathway.
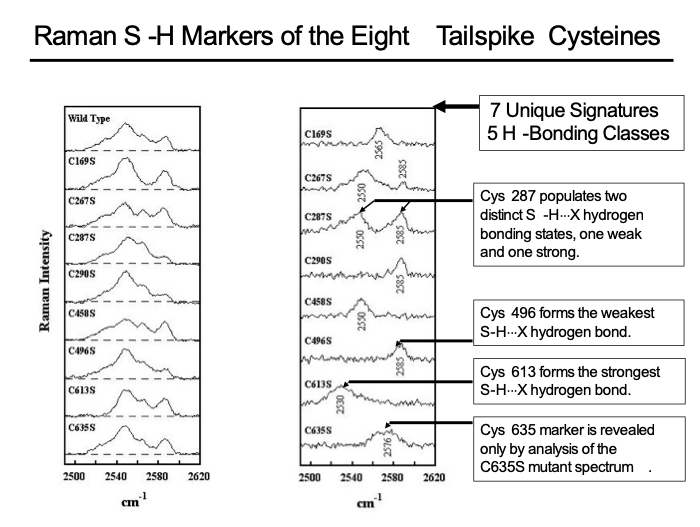
Figure 1. S–H Raman signatures (2480-2620 cm-1) of tailspike cysteines. Left Panel: The Raman S–H profiles observed for the wild-type tailspike and for each of the eight Cys → Ser mutants, as labelled. Right Panel: Raman difference spectra, computed as wild-type minus mutant, for each of the eight Cys → Ser mutants. In each trace, the S–H Raman signature of the mutated Cys site is revealed as a positive spectrum. Tailspike concentrations range from 81 to 127 μg/μL and other experimental conditions are given by Raso et al., 2001.
The significance of these results for the tailspike structure, stability and folding pathway have been discussed in detail by Raso et al., 2001. The strategy of connecting the unique strength of the Raman method to the tailspike crystal structure — caricatured in Figure 2 — provides a model for future applications that may gain new insights into protein folding and stability

Figure 2. A portrait of Sir C. V. Raman, discoverer of the spectroscopic effect bearing his name. The original photo has been modified to include the crystal structure of the trimeric P22 tailspike in Raman’s right hand.
Finally, I would like to conclude with a few observations about Jonathan King the man. Outside of the scientific milieu, Jon is clearly a devoted family man and a loyal friend. Over the years of our collaboration and friendship, my wife and I have gotten to know Jon and Jackie quite well. We continue to enjoy their companionship and share family stories when we get together on Cape Cod during our summer sojourns in Falmouth. Jon has always made time for us to socialize and has cheerfully conversed in topics of political and social importance. I am so grateful for having had the opportunity to know Jon, to work with him, to share in his research activities and to learn from him.
Best wishes for a long, healthy and well-deserved retirement.


References
Fish, S. R., Hartman, K. A., Fuller, M. T., King, J. and Thomas, G. J., Jr., “Investigations of Secondary Structures and Macromolecular Interactions in P22,” Biophys. J. 32:234-237, 1980.
Raso, S. W., Clark, P. L., Haase-Pettingell, C., King, J. and Thomas, G. J., Jr. “Distinct Cysteine Sulfhydryl Environments Detected by Analysis of Raman S-H Markers of Cys → Ser Mutant Proteins,” J. Mol. Biol. 307:899-911, 2001.
Sargent, D., Benevides, J. M., Yu, M.-H., King, J. and Thomas, G. J., Jr., “Secondary Structure and Thermostability of the Phage P22 Tailspike. XX. Analysis by Raman Spectroscopy of the Wild-Type Protein and a Temperature-Sensitive Folding Mutant,” J. Mol. Biol. 199:491-502, 1988.
Thomas, G. J., Jr., Li, Y., Fuller, M. T. and King, J., “Structural Studies of P22 Phage, Precursor Particles and Proteins by Laser Raman Spectroscopy,” Biochemistry 21:3866-3878, 1982.
Correspondence may be addressed to the author at his residential address: 134 Pine Street, Homosassa, FL 34446

